Abstract
Introduction
FXIII is a crucial transglutaminase enzyme which crosslinks fibrin polymers to increase the clot stiffness by 5-fold, thereby ensuring clot structure stabilization for efficient hemostasis. FXIII is activated by thrombin to form FXIIIa which in turn catalyzes the covalent bonds between fibrin alpha-gamma chains. FXIII has also been indicated to upregulate thrombin generation along with other coagulation factors. This interplay of FXIII and thrombin can have profound consequences on the resulting clot structure. Further, the role of FXIII in clot retraction is disputed. In this work, we investigated the effect of FXIII on thrombin generation and its effects on the structure of retracted clots by using FXIII inhibitors.
Methods
Phlebotomized platelet rich plasma (PRP) and platelet poor plasma (PPP) were isolated from healthy donors. PRP and PPP samples were incubated with varying doses of FXIII inhibitors (T101, Zedira GmBH) at RT for 15mins prior to performing thrombin generation experiments (Calibrated Automated Thrombogram). Similarly, clot retraction experiments were carried out with 20mM Ca2+ in 1 mL PRP in glass test tubes and incubated at 37°C. After 2 h, the clots were weighed, preserved using liquid nitrogen and methyl butyrate, and embedded in OCT. These clots were further sectioned into 5 um sections and stained for fibrinogen and PLT CD61. The sections were imaged using confocal microscopy and Transmission Electron Microscopy to study the structure and PLT-Fibrin interaction in retracted clots. The exuded serum after retraction was used to measure PLT concentration and D-Dimer levels to understand the relative incorporation of PLTs and fibrinolysis respectively. The effect of FXIII inhibitors on platelet aggregation was quantified using multiplate aggregometry. FXIII deficient plasma was used as a control.
Results
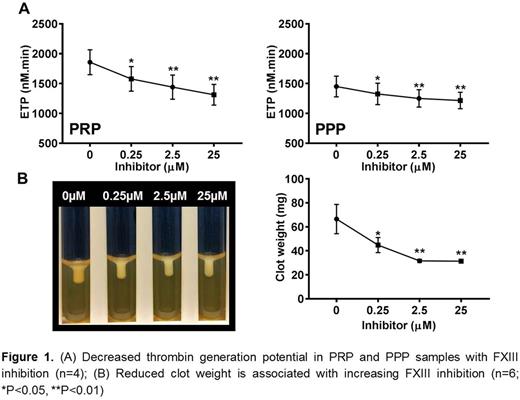
Inhibition of FXIII results in a drop in the total thrombin generation potential in PRP and PPP (Fig 1A), with a reduction in clot size and weight in the retracted clots (Fig 1B). FXIII inhibition does not affect platelet aggregation with ADP, platelet incorporation to clots and fibrinolysis (D-Dimers in exuded serum) under normal conditions. The structural studies reveal fibrin collapse, reduced clot size and weight, and increased plasma exclusion in FXIII inhibited clots.
Conclusion
Our data suggest that FXIII mediated crosslinking is important for thrombin generation and platelet-fibrin interaction for efficient clot retraction. The uncrosslinked fibrin collapse is due to higher local platelet contractile forces compared to the opposing fibrin tensile forces leading to abnormal retraction. The current work reveals the importance of force balances in clot structure and its resulting phenotypes associated with various pathologies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal